
Glutaban-IV, L-Alanyl-L-Glutamine Solution for Infusion (20%w/v)
December 8, 2025
AQABAN-AQ , Diclofenac Sodium Injection IP
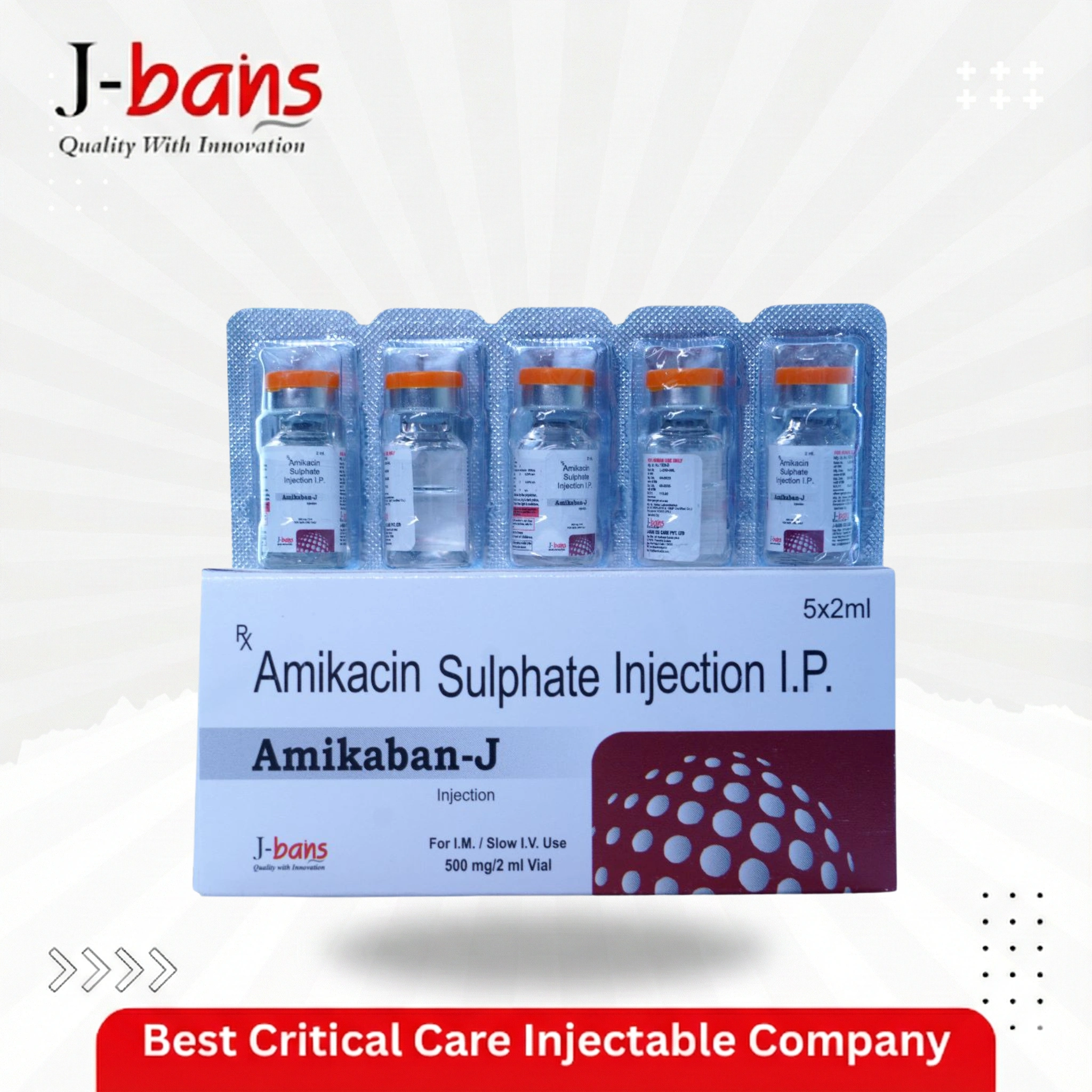
December 17, 2025AMIKABAN-J, Amikacin Sulphate Injection
₹113.00
AMIKABAN-J (Amikacin Sulphate Injection) from J-Bans Pharma stands as a critical last-line defense against multidrug-resistant Gram-negative pathogens. As a forward-thinking Critical Care PCD Pharma Company in India, we provide this potent aminoglycoside antibiotic to ICUs, burns units, and oncology wards where conventional therapies fail.
AMIKABAN-J is essential for treating life-threatening infections caused by Pseudomonas aeruginosa, Acinetobacter, and resistant Enterobacteriaceae. Through our dedicated PCD franchise network, J-Bans ensures this vital therapeutic weapon is available with unwavering quality and reliability, supporting clinicians in their most challenging battles against superbugs.
Product Overview
AMIKABAN-J is a sterile, clear, colorless solution of Amikacin Sulphate Injection IP, presented in a 2 ml vial containing 500 mg of amikacin (250 mg/ml). It is a semi-synthetic aminoglycoside derived from kanamycin A, specifically engineered to resist degradation by many bacterial enzymes that inactivate other aminoglycosides.
Manufactured under stringent aseptic conditions in our WHO-GMP certified facility, each batch undergoes rigorous testing for potency, sterility, and endotoxin levels, meeting the exacting standards of a premier Critical Care Injectable Company. The high-concentration formulation is optimized for both intramuscular and slow intravenous administration.
Therapeutic Indications
Severe Hospital-Acquired Infections: Ventilator-associated pneumonia (VAP), hospital-acquired pneumonia (HAP), bacteremia.
Complicated Intra-abdominal Infections: Often in combination with an anti-anaerobic agent.
Complicated Urinary Tract Infections: Including pyelonephritis caused by multi-drug resistant organisms.
Infections in Neutropenic Patients: Febrile neutropenia where Gram-negative coverage is essential.
Mycobacterial Infections: Second-line treatment for multidrug-resistant tuberculosis (MDR-TB).
Burn Wound Infections: For Gram-negative coverage in severe burn sepsis.
Endocarditis: Caused by susceptible Gram-negative organisms (in combination).
Dosage and Administration
Once-Daily Dosing (ODD) Regimen (Preferred): 15-20 mg/kg administered once every 24 hours by IV infusion over 30-60 minutes or IM injection. This maximizes efficacy and may reduce toxicity.
Traditional Multiple Daily Dosing: 7.5 mg/kg every 12 hours (or 5 mg/kg every 8 hours). LESS COMMONLY USED NOW.
IV Administration: MUST be diluted in 50-100 ml of compatible IV fluid (Normal Saline or D5W) and infused over 30-60 minutes.
IM Administration: For deep intramuscular injection into a large muscle mass.
CRITICAL – Therapeutic Drug Monitoring (TDM):
Peak Level (drawn 30 minutes after end of 30-min infusion): Target 20-30 mg/L for serious infections; 35-45 mg/L for MDR-TB or cystic fibrosis.
Trough Level (drawn just before next dose): Should be <5 mg/L to minimize nephrotoxicity and ototoxicity. For ODD, a trough <1 mg/L is often targeted.
Renal Impairment: DOSE ADJUSTMENT IS MANDATORY. Dosing interval is extended based on creatinine clearance (CrCl). Use formulas (e.g., Hartford nomogram) or estimate: Interval (hours) = Serum Creatinine (mg/dL) × 9. Monitor levels closely.
Clinical Advantages
Broad Spectrum Against Resistant Gram-negatives: Effective against many strains resistant to gentamicin and tobramycin.
Concentration-Dependent Killing: Supports efficient once-daily dosing, improving patient convenience and potentially reducing toxicity.
Synergy with Beta-Lactams: Combined with drugs like piperacillin-tazobactam or carbapenems for synergistic effect against Pseudomonas and other pathogens.
Quality-Assured Manufacturing: Produced by J-Bans, a Critical Care PCD Pharma Company committed to supplying essential anti-infectives with guaranteed sterility and potency.
Last-Resort Option: A vital component in the antibiotic arsenal when other options are exhausted.
Safety Profile and Contraindications
WARNING: NEPHROTOXICITY and OTOTOXICITY. Risk is increased with prolonged use (>5 days), high trough levels, concomitant use of other nephro/ototoxic drugs (vancomycin, loop diuretics), dehydration, and renal impairment.
Contraindications: Hypersensitivity to amikacin or other aminoglycosides. Myasthenia gravis (can cause neuromuscular blockade).
Serious Adverse Effects:
Ototoxicity: Irreversible damage to vestibular and/or auditory function (tinnitus, hearing loss, vertigo).
Nephrotoxicity: Non-oliguric renal failure, elevated serum creatinine.
Neuromuscular Blockade: Can potentiate muscle relaxants, leading to respiratory depression.
Precautions: Use with extreme caution in the elderly, neonates, and patients with pre-existing renal, auditory, or vestibular dysfunction. Ensure adequate hydration. Avoid concurrent use of other nephrotoxic/ototoxic drugs unless absolutely necessary.
Storage and Handling
Store below 25°C. Protect from light. Do not freeze.
The solution should be clear and colorless. Do not use if discolored or precipitated.
Once diluted for infusion, use within 24 hours when stored at room temperature or as per manufacturer’s instructions.
Do not mix physically with beta-lactam antibiotics (e.g., penicillins, cephalosporins) in the same syringe or IV bag due to mutual inactivation. Administer separately.
Packaging Information
Primary Packaging: 2 ml clear glass vial with rubber stopper and aluminum seal, containing Amikacin Sulphate IP 500 mg/2 ml.
Secondary Packaging: Individual carton, often in packs of 1 vial.
Labeling: Prominent warning: “NEPHROTOXICITY and OTOTOXICITY. Therapeutic drug monitoring essential.” Clear display of brand name AMIKABAN-J and strength.
Why Partner with J-Bans for High-Alert Antibiotics?
Marketing specialized, high-alert antibiotics like AMIKABAN-J requires technical expertise and trust. Partnering with J-Bans Pharma, a Critical Care PCD Pharma Company in India, provides:
Clinical Credibility: We supply partners with detailed TDM protocols, dosing nomograms, and susceptibility data.
Focus on Safety: We emphasize the critical importance of monitoring, positioning our partners as responsible suppliers.
Niche Market Access: This product opens doors to hospital microbiology departments and infectious disease specialists.
Comprehensive Support: From technical data sheets to training on aminoglycoside pharmacokinetics, we empower our partners.
FAQs for AMIKABAN-J
Q1: Why is Therapeutic Drug Monitoring (TDM) absolutely mandatory for AMIKABAN-J?
A: Aminoglycosides like amikacin have a narrow therapeutic index—the difference between an effective dose and a toxic dose is small. TDM ensures:
Efficacy: Peak levels are high enough to kill bacteria (concentration-dependent killing).
Safety: Trough levels are kept low to minimize the risk of irreversible kidney and ear damage. Dosing without TDM is dangerous and substandard.
Q2: What is the advantage of Once-Daily Dosing (ODD) over multiple daily doses?
A: ODD (15-20 mg/kg once daily) takes advantage of amikacin’s concentration-dependent killing and post-antibiotic effect. It achieves higher peak levels for better bacterial kill, may reduce the risk of adaptive resistance, and can be more convenient. It also allows for lower trough levels, potentially reducing nephrotoxicity.
Q3: How does J-Bans support its PCD partners in promoting a complex drug like AMIKABAN-J?
A: As a specialized Critical Care PCD Pharma Company, we provide:
TDM Kits & Nomograms: Ready-to-use tools for hospital pharmacists and doctors.
Infection Management Guides: Highlighting its role in specific MDR infections.
Access to Medical Information: For complex cases or dosing questions.
Training on Pharmacokinetics: Helping partners speak confidently with intensivists and microbiologists.
Q4: Can AMIKABAN-J be used to treat tuberculosis?
A: Yes, it is a Group C second-line injectable agent for the treatment of Multidrug-Resistant Tuberculosis (MDR-TB). In such cases, it is used in prolonged regimens (6-8 months of injectable phase) under direct specialist supervision, with specific dose adjustments and intensive monitoring for ototoxicity.
Q5: What should be done if a patient shows signs of ototoxicity (tinnitus, hearing loss)?
A: Immediately discontinue AMIKABAN-J and obtain an audiometric evaluation. Ototoxicity can be irreversible. The risk-benefit ratio of continuing therapy must be re-evaluated by the treating physician. Alternative antibiotics should be sought.
Q6: How is the dose calculated for a patient with renal impairment?
A: Two main methods:
Interval Extension: Keep the same dose (e.g., 15 mg/kg) but extend the interval based on CrCl (e.g., CrCl 40-60: every 24h; CrCl 20-40: every 36h; CrCl <20: every 48h). TDM is still essential.
Dose Reduction: Reduce the dose while keeping a 24-hour interval. This is less common.
A pharmacist or clinical pharmacologist should be involved in dose calculations for renally impaired patients.
Q7: Is it safe to use during pregnancy?
A: Category D. Aminoglycosides cross the placenta and there is a risk of fetal ototoxicity. Should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus, typically only for life-threatening infections with no safer alternative.
Q8: What is the shelf life of AMIKABAN-J?
A: The shelf life is 24 months from the date of manufacture when stored in the original packaging below 25°C, protected from light.
Related products
Meropenem & Sulbactam
₹2,150.00



Reviews
There are no reviews yet.